-
New transformation development of styrenes by dealkenylation
2020-11-20
As a fundamental bulk chemical, styrene has been widely used in chemical synthesis, as well as in areas such as plastics and elastomers. Industrially, it could be produced through the conversion of hydrocarbon derived from petroleum and natural gas, starting from benzene and ethylene through the intermediacy of ethylbenzene followed by the dehydrogenation. In light of the availability and significance of styrene chemistry, both olefin metathesis reaction and Heck reaction based on alkene have been awarded Nobel prize in 2005 and 2010, respectively. Moreover, many efforts have been contributed to develop other functionalization of styrene in the past decades, such as traditional wacker oxidation, alkene difunctionalization, etc. These significant methodologies rely heavily on the C=C double bond, the C(sp2)-H bond, and the allylic C(sp3)-H bond functionalization. Alternatively, the ongoing discovery of new bond disconnection logic based on vinyl arenes has been a sought-after object for related chemists.
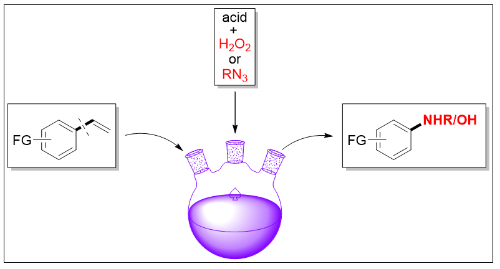
Along these lines, the breakthrough result, published by scientist from Peking university, State Key Laboratory of Natural and Biomimetic Drugs proposes a selective dealkenylative strategy to realize the C(Ar)-C(alkenyl) single bond cleavage (the Bond Dissociation Energy of the C(Ar)-C(alkenyl) single bond is 116.9 kcal/mol) generating a series of site-specific and value-added oxygen- and nitrogen-moiety decorated aromatics, which would otherwise be difficult to access. As documented, this is the first transformation of vinyl arenes via dealkenylative C-C single bond cleavage, uncovering the new activation mode of alkene chemistry and thus driving further methodology development based on alkene(Research, Volume 2020, Article ID 7947029). https://spj.sciencemag.org/journals/research/2020/7947029/
Crucial to the success of this reaction is to avoid the inherent sensitive challenge of traditional C=C double bond cleavage leading to the corresponding aldehyde or ketone derivatives. Inspired by the traditional Hock process and Schmidt reaction, the researchers proposed a cascade activation strategy via the initial C=C double bond pre-activation to break the conjugate structure of styrene and generate the active intermediate for the subsequent C(Ar)-C1 bond cleavage. Accordingly, a system involving the Markovnikov’s hydrofunctionalization of alkene to generate the desired intermediate was developed to realize the ensuing C(Ar)-C(alkenyl) single bond cleavage to form phenols and arylamines derivatives. The transition-metal free and redox-neutral nature of this protocols enabled it applicable to the late-stage modification of biologically relevant molecules, sourcing the nitrogen and oxygen atom from the readily available azide reagents and aqueous hydrogen peroxide solution. Compared to the poor regioselectivity and limited substrate scope in the typical aromatic C-H amination and hydroxylation process, this chemistry features site-directed selectivity and broad substrate scope. The authors hope that the present cascade activation strategy will inspire further methodology development based on olefins.
Tag: Emerging materials research